Business opportunities for treatment of cutaneous warts.
Superior ratio: Clinical benefit vs Risk for Corrosion!
A patent is for sale through direct assignment or licensing.
The patent covers mild removal of cutaneous warts.
No clinical inferiority in treatmenf of warts and no irritation compared to corrosive market leader (ISO 10993-10:2010).
►Clinical Investigation
►Clinical Evaluation►Biological Evaluation
►Draft Scientific Report
►Stability, suppliers etc
Annoying Warts
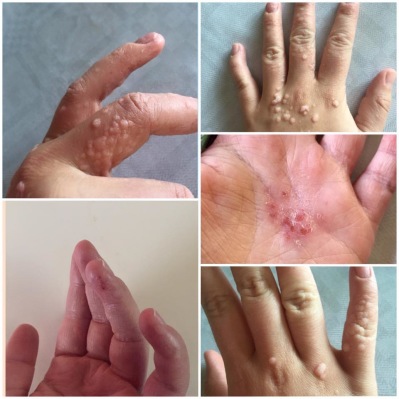
Viral warts are a common skin disease, most frequently affecting the hands and feet, caused by the human papilloma virus (HPV).
Warts on the soles of the feet are also called 'plantar warts' or 'verrucas' The prevalence of warts or verrucae are reported to be 3.3% in adults aged 15 to 75 years, and are up to 24% in 16–18 year olds.1
Although most plantar warts will spontaneously disappear without treatment, many patients seek treatment for a variety of reasons. They can be large and painful in the plantar area, cosmetically unsightly on e.g., face, hands, and spread to distant anatomical regions by autoinoculation. They also provide a significant reservoir of HPV infection in the community.
Treatments consists mainly of self-care products, such as topical painting of warts with acids and freezing techniques. Formic acid 85% solution (brands VårtFri, EndWarts, ZeroVerruce) is reported to clear the skin in ca 90% of the patients in 3-4 weeks treatment compared to 5-10% in the placebo group.2, 3.
Other brands include e.g., Verruxin (salicylic acid), Wartner and Wortie (trichloroacetic acid TCA).
Patent
Read more at EPO
Applicants
Inventors
LODÉN, Marie, SWAHN, Britt-Marie
Agents
BRANN AB
Priority Data
18175098.530.05.2018EP
The efficacy and safety of Patented Wart Solution.
In a prospective post-market, controlled, randomized clinical study with concealed allocation the efficacy and safety of Patented Wart Solution and the market leader were compared (n=76). The patients reported the results using a web-based questionnaire and by submission of digital photographs.
Superiority of the comparator was anticipated, but no significant difference in efficacy between the two products, neither in the Full Analysis Set (FAS) population, nor in the Per Protocol Set (PPS) population was found. However, clearing of warts was associated with ulcers and infections during treatment with the comparator.
The Patented Wart Solution was confirmed as mild and is a non-irritant, whereas the market leader is known to be corrosive due to its content of formic acid.
Therefore, the Patented Wart Solution is safely used without risks for corrosion, also in e.g. children, whereas the formic acid product must be handled with great care, also by adults.
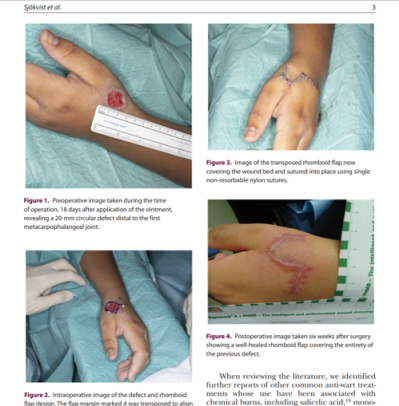
Case reports on corrosion are also reported, for example, a 20-mm circular full-thickness defect was noted on the dorsum of the hand in a 11-year-old girl after treatment with formic acid. She required surgical debridement and local flap coverage.5
Ingredients
►Water - Solvent
►Zinc sulfate heptahydrate - Protecting astringent
►Methyl salicylate - Minty, fresh odour
►Oleth-20 - Stabiliser
References
1. Kwok CS, Gibbs S, Bennett C, Holland R, Abbott R. Topical treatments for cutaneous warts. Cochrane Database Syst Rev 2012(9):Cd001781.
2. Kilkenny M, Merlin K, Young R, Marks R. The prevalence of common skin conditions in Australian school students: 1. Common, plane and plantar viral warts. Br J Dermatol 1998;138(5):840-5.
3. Faghihi G, Vali A, Radan M, Eslamieh G, Tajammoli S. A double-blind, randomized trial of local formic acid puncture technique in the treatment of common warts. Skinmed 2010;8(2):70-1.
4. Bhat RM, Vidya K, Kamath G. Topical formic acid puncture technique for the treatment of common warts. Int J Dermatol 2001;40(6):415-9.
5. Sjökvist O, Smolle C, Jensson D, Huss F. A full-thickness chemical burn to the hand using formic acid-based anti-wart treatment: a case report and literature review. Scars Burn Heal 2020;6:2059513119897888.
Note: Text based on automatic Optical Character Recognition processes. Please use the PDF version for legal matters[EN ]
CLAIMS
1. A topical composition comprising: an alpha hydroxy acid selected from the group consisting of lactic acid, malic acid, and glycolic acid, and combinations thereof in a total amount of from 50 to 80 % by weight; 0.1 to 10 % by weight of methyl salicylate; and, a wa ter soluble zinc salt in an amount of from 0.2 to 4% by weight of the formulation calculated as Zn.
2. The composition of claim 1, further comprising a solubiliser in an amount effective for solubilizing the methyl salicylate in the formulation.
3. The composition of claim 2, wherein said solubiliser is an ethoxylated fatty alcohol.
4. The composition of any one of the preceding claims, wherein the the total amount of the alpha hydroxy acid is within the range of 50-70 % by weight.
5. The composition of any one of the preceding claims, wherein the amount of methyl salicylate is 0.2-10% by weight.
6. The composition of any one of the preceding claims, wherein the alpha hydroxy acid is glycolic acid.
7. The composition of any one of the preceding claims, wherein the water soluble zinc salt is one or more members selected from the group consisting of zinc sulphate, zinc chlo ride, zinc acetate, zinc glycolate, zinc gluconate, zinc carbonate, zinc oxide, zinc oleate, zinc stearate, zinc propionate, zinc salicylate, zinc lactate, zinc coceth sulphate, zinc citrate, zinc ascorbate and zinc glutamate.
8. The composition of claim 7, wherein the water soluble zinc salt is zinc sulphate.
9. The composition according to any one of the preceding claims, wherein the amount by weight of zinc is from 1.0 to 2.5%, glycolic acid is from 55 to 65 %, and methyl salicylate is from 2 to 5 %, respectively.
10. A kit comprising a container holding the composition of any one of claims 1-9, and an applicator for topical application of the composition.
11. An applicator comprising a felt tip, a brush tip, or a roller ball tip attached to a con-tainer holding the composition of any one of claims 1-9, from which container the composi-tion can be delivered to the tip of the applicator.
12. A pen containing the composition of any one of claims 1-9 for the topical administra tion of the composition from a tip of the pen to an affected skin area for the treatment of warts.
13. A method of treating warts wherein the composition of any one of claims 1-9 is ap plied to an affected skin area of a subject.
14. A method of treating of treating fungal infections of the toenails or fingernails, wherein the inventive composition of any one of claims 1-9 is applied topically to an area of affected nail.