Business opportunities for Fungy Nails
Superior ratio: Clinical benefit vs risks for adverse events and antifungal resistance
A patent is for sale through direct assignment or licensing.
The patent covers mild removal of nail fungus.
Non-touch felt-tip pen easy and hygienic to use, with fresh minty odour.
Patented Nail Treatment shows clinical superiority in a randomized Postmarket Clinical Follow-up study.
No drug resistance and no skin irritation in a human patch test (ISO 10993-10:2010).
Fungy Nails
Infections in the nails by fungi affects 2–18% or more of the world’s population. The market revenue for topical treatment is estimated to US dollar 422 Mil (IQVIA Database, 2022). The prevalence of onychomycosis increases with age, and 1 out of 5 persons are affected!
The reasons for the age-dependent prevalence may be poor peripheral circulation, smoking habits, diabetes, nail trauma, long exposure to pathogenic fungi, suppressed immune functions and inability to maintain good foot care.
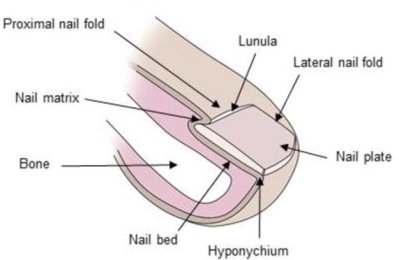
The thickness and hydrophilicity of the nail plate makes it a formidable barrier to anti-fungal drug delivery. The size of drug molecules, such as terbinafine, ciclopirox, efinaconazole and tavaborole is very large, making it extremely difficult to achieve effective concentrations in the infected area. The complete cure for the drug products is reported to be only beetween 4% and 15% (Cochrane).
Therefore, the present substance-based medical device using the several times smaller glycolic acid is a better option than the drug molecules. Acidic environments are hostile to fungi!
Medical Devices use a great variety of substances with unknown efficacy. Historically, Conformacy assessment by a Notified Body may not have been performed, putting many of them at risk with the new stringent Medical Device Regulation. Some of them may also be classified as Class III devices according to the MDR due to their contents of drug molecules.
What is antifungal resistance?
Antifungal resistance occurs when an antifungal medication no longer works to treat a fungal infection. The fungus can fight off the medicine’s effects.
Drug resistance is a serious global health problem. It greatly limits treatment options. If a fungus doesn’t respond to one of three available classes of antifungal drugs, the other options may be less effective. Some drugs cause more severe, potentially toxic side effects.
The increase in fungal resistance to e.g. terbinafin is an emerging finding, making CE-device a safer option, see below:
The efficacy and safety of Patented Nail Treatment.

The performance of Patented Nail Treatment has been evaluated in a postmarket clinical follow-up (PMCF) study using an internationally established medical device as comparator.
The PMCF-study was randomized (concealed allocation), web-based and using patient-reporting outcomes, including photographs and DNA-test for detection of dermatophytes.
100 patients were included. The results demonstrated that our formulation delivers superior outcomes, with approximately three times as many participants showing visible improvement in their nails compared to those using the leading competitor's product (blinded evaluation). Additionally, twice as many participants were free from fungi, as confirmed by DNA testing. Furthermore, complete curing (less than 10% residual involvement of the nail plate) was more prevalent with our formulation. No serious and no unexpected adverse events were observed.
Ingredients in Patented Nail Treatment
►Water - Solvent
►Zinc sulfate heptahydrate - Protecting astringent
►Methyl salicylate - Minty, fresh odour
►Oleth-20 - Stabiliser


Note: Text based on automatic Optical Character Recognition processes. Please use the PDF version for legal matters[EN ]
CLAIMS
1. A topical composition comprising: an alpha hydroxy acid selected from the group consisting of lactic acid, malic acid, and glycolic acid, and combinations thereof in a total amount of from 50 to 80 % by weight; 0.1 to 10 % by weight of methyl salicylate; and, a wa ter soluble zinc salt in an amount of from 0.2 to 4% by weight of the formulation calculated as Zn.
2. The composition of claim 1, further comprising a solubiliser in an amount effective for solubilizing the methyl salicylate in the formulation.
3. The composition of claim 2, wherein said solubiliser is an ethoxylated fatty alcohol.
4. The composition of any one of the preceding claims, wherein the the total amount of the alpha hydroxy acid is within the range of 50-70 % by weight.
5. The composition of any one of the preceding claims, wherein the amount of methyl salicylate is 0.2-10% by weight.
6. The composition of any one of the preceding claims, wherein the alpha hydroxy acid is glycolic acid.
7. The composition of any one of the preceding claims, wherein the water soluble zinc salt is one or more members selected from the group consisting of zinc sulphate, zinc chlo ride, zinc acetate, zinc glycolate, zinc gluconate, zinc carbonate, zinc oxide, zinc oleate, zinc stearate, zinc propionate, zinc salicylate, zinc lactate, zinc coceth sulphate, zinc citrate, zinc ascorbate and zinc glutamate.
8. The composition of claim 7, wherein the water soluble zinc salt is zinc sulphate.
9. The composition according to any one of the preceding claims, wherein the amount by weight of zinc is from 1.0 to 2.5%, glycolic acid is from 55 to 65 %, and methyl salicylate is from 2 to 5 %, respectively.
10. A kit comprising a container holding the composition of any one of claims 1-9, and an applicator for topical application of the composition.
11. An applicator comprising a felt tip, a brush tip, or a roller ball tip attached to a con-tainer holding the composition of any one of claims 1-9, from which container the composition can be delivered to the tip of the applicator.
12. A pen containing the composition of any one of claims 1-9 for the topical administra tion of the composition from a tip of the pen to an affected skin area for the treatment of warts.
13. A method of treating warts wherein the composition of any one of claims 1-9 is ap plied to an affected skin area of a subject.
14. A method of treating of treating fungal infections of the toenails or fingernails, wherein the inventive composition of any one of claims 1-9 is applied topically to an area of affected nail.
RELATED PATENT DOCUMENTS
ApplicantsEVIDERM INSTITUTE AB [SE]/[SE]
Inventors
LODÉN, Marie
SWAHN, Britt-Marie
Agent
BRANN AB
Priority Data
18175098.530.05.2018 EP
Publication LanguageEnglish (en)
Filing LanguageEnglish (en)
References
1. Kwok CS, Gibbs S, Bennett C, Holland R, Abbott R. Topical treatments for cutaneous warts. Cochrane Database Syst Rev 2012(9):Cd001781.
2. Kilkenny M, Merlin K, Young R, Marks R. The prevalence of common skin conditions in Australian school students: 1. Common, plane and plantar viral warts. Br J Dermatol 1998;138(5):840-5.
3. Faghihi G, Vali A, Radan M, Eslamieh G, Tajammoli S. A double-blind, randomized trial of local formic acid puncture technique in the treatment of common warts. Skinmed 2010;8(2):70-1.
4. Bhat RM, Vidya K, Kamath G. Topical formic acid puncture technique for the treatment of common warts. Int J Dermatol 2001;40(6):415-9.
5. Baswan S, Kasting GB, Li SK, et al. Understanding the formidable nail barrier: A review of the nail microstructure, composition and diseases. Mycoses 2017;60(5):284-95.
6. Foley K, Gupta AK, Versteeg S, et al. Topical and device-based treatments for fungal infections of the toenails. Cochrane Database Syst Rev 2020;1:Cd012093.